For people living with rare diseases, accessing the right medication can sometimes feel like navigating a maze. One common challenge arises when a medication is approved for a new indication.
A new indication means that the FDA has officially approved a medication to treat a condition it wasn’t originally prescribed for. This often happens after clinical studies show that a drug is effective for a different disease or a different patient population. While this is great news for patients, it can sometimes create delays in insurance coverage.
Even after FDA approval, insurance companies may take several months to update their systems to reflect the new indication. During this time, coverage can be inconsistent or denied, which can be frustrating and stressful.
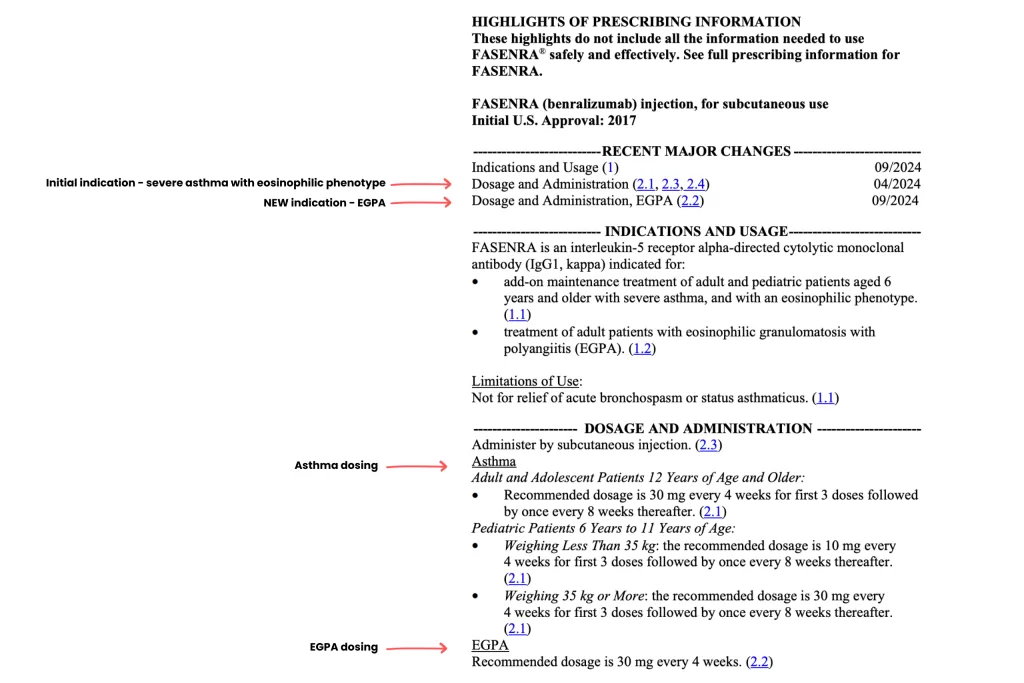
One way to smooth the process is to provide your physician and insurance team with the FDA-approved product information sheet. This sheet includes the specific disease the medication is approved to treat and the recommended dosing. Because it is published at the time of FDA approval, it is always available and can be a critical tool to support your access to treatment.
By understanding what a new indication means and leveraging official documentation, patients and healthcare teams can better navigate insurance challenges and ensure timely access to the therapies they need.
You can share this information with your doctor. On the product information sheets you will find the indication(s) (i.e. EGPA) and dosing for the indication (30 mg once every 4 weeks.
This information can be used by your doctor to assist with getting prior authorization for your medications as well as ensure you are getting the proper dosing.

TAVNEOS for GPA and MPA Vasculitis